 This is one of the latest advancements in the polymer science.
This is one of the latest advancements in the polymer science.
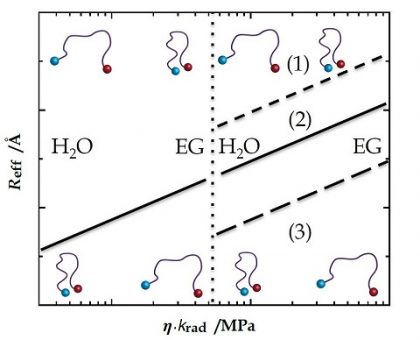
A flexible peptide chain displays structural and dynamic properties that correspond to its folding and biological activity. These properties are mirrored in intrachain site-to-site distances and diffusion coefficients of mutual site-to-site motion. Both distance distribution and diffusion determine the extent of Förster resonance energy transfer (FRET) between two sites labeled with a FRET donor and acceptor. The relatively large Förster radii of traditional FRET methods (R0 > 20 Å) lead to a fairly low contribution of diffusion. We introduced short-distance FRET (sdFRET) where Dbo, an asparagine residue conjugated to 2,3-diazabicyclo[2.2.2]octane, acts as acceptor paired with donors, such as naphtylalanine (NAla), tryptophan, 5-l-fluorotryptophan, or tyrosine. The Förster radii are always close to 10 Å, which makes sdFRET highly sensitive to diffusional motion. We recently found indications that the FRET enhancement caused by diffusion depends symmetrically on the product of the radiative fluorescence lifetime of the donor and the diffusion coefficient. In this study, we varied this product by two orders of magnitude, using both donors of different lifetime, NAla and FTrp, as well as a varying viscogen concentration, to corroborate this statement. We demonstrate the consequences of this relationship in evaluating the impact of viscogenic coadditives on peptide dimensions.
1. Introduction
- Förster, T. Zwischenmolekulare Energiewanderung und Fluoreszenz. Ann. Phys. 1948, 2, 55–75. [Google Scholar] [CrossRef]
- Stryer, L.; Haugland, R.P. Energy Transfer: A Spectroscopic Ruler. Proc. Natl. Acad. Sci. USA 1967, 58, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Haas, E. Fluorescence Resonance Energy Transfer (FRET) and Single Fluorescence Detection Studies of the Mechanism of Protein Folding and Unfolding. In Protein Folding Handbook; Buchner, K., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Kluwer Academic/Plenum: New York, NY, USA, 2006. [Google Scholar]
- Stryer, L. Fluorescence Energy Transfer as a Spectroscopic Ruler. Annu. Rev. Biochem. 1978, 47, 819–846. [Google Scholar] [CrossRef] [PubMed]
- Stryer, L.; Thomas, D.D.; Meares, C.F. Diffusion-Enhanced Fluorescence Energy Transfer. Annu. Rev. Biophys. Bioeng. 1982, 11, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Ha, T. Single-Molecule Fluorescence Resonance Energy Transfer. Methods2001, 25, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Schuler, B. Single-Molecule Fluorescence Spectroscopy of Protein Folding. ChemPhysChem 2005, 6, 1206–1220. [Google Scholar] [CrossRef] [PubMed]
- Schuler, B. Application of Single Molecule Förster Resonance Energy Transfer to Protein Folding. Methods Mol. Biol. 2007, 350, 115–138. [Google Scholar] [PubMed]
- Sahoo, H.; Roccatano, D.; Hennig, A.; Nau, W.M. A 10-Å Spectroscopic Ruler Applied to Short Polyprolines. J. Am. Chem. Soc. 2007, 129, 9762–9772. [Google Scholar] [CrossRef] [PubMed
************************
Why Should Be “A Paid-Subscriber” and “Advertiser”
Keeping an independent media in countries that impose limitations on self supporting media, will help to support the humankind’s freedom. If you believe it, please act to be a PRO-MEMBER by clicking “HERE“, or:
Please send your PR’s directly to my email address to be published in the world via ” http://pimi.ir ” my address is: aasaatnia@live.com