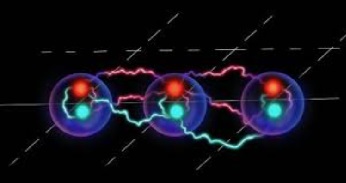
Based on the CBS news broadcasting, Researchers at Rice University located in the Texas State have made a meaningful advance in the simulation of molecular electron transfer—a fundamental process underpinning countless physical, chemical and biological processes. (Following Image) The study, published in Science Advances, details the use of a trapped-ion quantum simulator to model electron transfer dynamics with unprecedented tunability, unlocking new opportunities for scientific exploration in fields ranging from molecular electronics to photosynthesis.
Electron transfer, critical to processes such as cellular respiration and energy harvesting in plants, has long posed
challenges to scientists due to the complex quantum interactions (Front page Image) involved. Current computational techniques often fall short of capturing the full scope of these processes. The multidisciplinary team at Rice, including physicists, chemists and biologists, addressed these challenges by creating a programmable quantum system capable of independently controlling the key factors in electron transfer: donor-acceptor energy gaps, electronic and vibronic couplings and environmental dissipation.
Using an ion crystal trapped in a vacuum system and manipulated by laser light, the researchers demonstrated the ability to simulate real-time spin dynamics and measure transfer rates across a range of conditions. The findings not only validate key theories of quantum mechanics but also pave the way for novel insights into light-harvesting systems and molecular devices.
“This is the first time that this kind of model was simulated on a physical device while including the role of the environment and even tailoring it in a controlled way,” said lead researcher Guido Pagano, assistant professor of physics and astronomy. “It represents a significant leap forward in our ability to use quantum simulators to investigate models and regimes that are relevant for chemistry and biology. The hope is that by harnessing the power of quantum simulation, we will eventually be able to explore scenarios that are currently inaccessible to classical computational methods.”
The team achieved a significant milestone by successfully replicating a standard model of molecular electron transfer using a programmable quantum platform. Through the precise engineering of tunable dissipation, the researchers explored both adiabatic and nonadiabatic regimes of electron transfer, demonstrating how these quantum effects operate under varying conditions. Additionally, their simulations identified optimal conditions for electron transfer, which parallel the energy transport mechanisms observed in natural photosynthetic systems.

“Our work is driven by the question: Can quantum hardware be used to directly simulate chemical dynamics?” Pagano said. “Specifically, can we incorporate environmental effects into these simulations as they play a crucial role in processes essential to life such as photosynthesis and electron transfer in biomolecules? Addressing this question is significant as the ability to directly simulate electron transfer in biomolecules could provide valuable insights for designing new light-harvesting materials.”
More information: Visal So et al, Trapped-ion quantum simulation of electron transfer models with tunable dissipation, Science Advances (2024). DOI: 10.1126/sciadv.ads8011
Provided by Rice University
This story was originally published on Phys.org. Subscribe to our newsletter for the latest sci-tech news updates.